genOway’s mouse models enable a translatable in vivo efficacy testing of therapies targeting the cGAS-STING pathway
What sets the genO-hcGAS mouse model apart?
Key features:
- Physiological regulation and expression of human cGAS
- Fully functional mouse immune system
- Lack of expression of the murine target gene
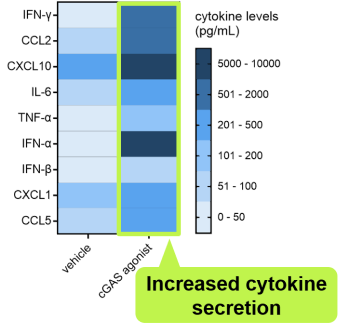
The genO-hcGAS mouse was used to test the efficacy of a cGAS agonist and cytokine production was observed.
Treatment with a cGAS agonist leads to cytokine secretion in genO-hcGAS mice
What sets the genO-hSTING mouse model apart?
Key features:
- Physiological regulation of the human STING
- Fully functional mouse immune system
- Lack of expression of the murine target gene
- Different versions of genO‑hSTING models are available (expressing different variants):

Curadev used the genO-hSTING mouse to test the efficacy of CRD5500, a STING agonist, for inhibiting pancreatic cancer development and observed tumor growth inhibition:
Efficacy assessment of a STING agonist in genO-hSTING mice

Adapted from Curadev's AACR 2022 poster, Banerjee et al.
The double-humanized genO-hcGAS/hSTING mouse model
The upgraded genO-hcGAS/hSTING mouse model resulting from the intercrossing of the genO-hcGAS and genO-hSTING models will be available in 2026, offering increased translatability.


Discover our available cell lines for syngeneic studies in immuno-oncology
As part of our catalog, we have a range of different tumor cell lines that can be used for performing efficacy assays in syngeneic models. Each cell line is extensively validated based on expression of the target protein and reporter cassette, as well as in tumor growth studies:
- genO-MC38-hSTING MRP R232-LZ
- genO-B16F10-hSTING MRP R232-LZ
Drive innate immune therapies forward with our genO-hcGAS & genO-hSTING models
Get in touch about
Let us know how we can help

Scientific excellence
From model design to experimental results
Featured in 600+ scientific articles

Collaborative approach
Collaboration with 17 Top Pharmas,
170+ Biotechs and 380+ Academic Institutions

Robust validation data on catalog models
Generated with biopharma partners and in-house

Innovative technologies
and guaranteed freedom to operate

Easy access to models
Models with certified health status from professional breeders in US and Europe