FcγR humanized mouse models enabling efficacy and PK/PD of therapeutic antibodies
hFcγR mice for therapeutic antibody assessment
Background
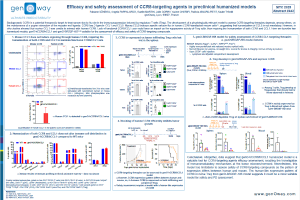
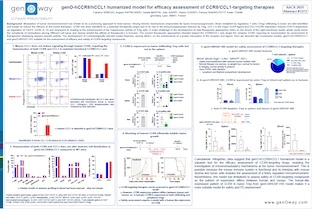
Enhanced activity of therapeutic IgG can be achieved by the modulation of Fc binding to Fcγ receptors (FcγR) which consequently modulate the Fc-effector function triggered upon crosslinking of target and effector cells by these antibodies. Assessment of therapeutic antibodies’ pharmacodynamics in preclinical models is challenging, as the FcγR differ between mice and human, as well as in their expression pattern. Humanized FcγR (hFcγR) model features a human-like expression pattern, in which humanized FcγRI, FcγRIIA, FcγRIIB, FcγRIIIA, FcγRIIIB are expressed and replace their mouse orthologs. We have previously reported that humanized FcγR expression pattern in hFcγR mice is consistent with their expression on human PBMC, with a few exceptions. Test of antibody-dependent cell cytotoxicity (ADCC) ex vivo in NK cells showed that Rituximab, an anti-hCD20 chimeric IgG1 with regular Fc, induces higher ADCC efficacy in NK cells from hFcγR than in NK cells from WT mice. Obinutuzumab, which also targets hCD20, but features an optimized Fc portion to enhance binding to FcγRIII, showed higher tumor cell lysis than Rituximab in NK cells from hFcγR mice, suggesting this model enables the ranking of antibodies.

Scientific excellence
From model design to experimental results
Featured in 600+ scientific articles

Collaborative approach
Collaboration with 17 Top Pharmas,
170+ Biotechs and 380+ Academic Institutions

Robust validation data on catalog models
Generated with biopharma partners and in-house

Innovative technologies
and guaranteed freedom to operate

Easy access to models
Models with certified health status from professional breeders in US and Europe