Novel TFRC humanized mouse model for drug delivery to the brain
Drug delivery to the brain (TFRC)
Abstract
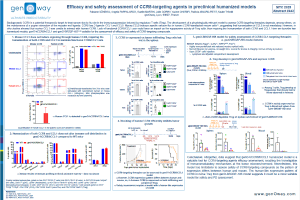
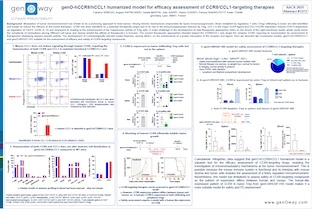
Gliomas are the most common neoplasm in the central nervous system (CNS). Although immunotherapy has shown efficacy in several tumor types, no immunotherapies are approved by the US Food and Drug Administration for treatment of gliomas. One of the biggest challenges in using immunotherapies in the CNS is the existence of the blood-brain barrier (BBB), which limits drug delivery and the subsequent therapeutic drug efficacy. In addition to biodistribution, assessment of PK in preclinical models do not reflect the clinical findings. Conjugation of therapeutic agents with molecules binding to TRFC expressed along the BBB are being used as one of the alternative approaches to deliver drugs into the CNS. Herein, we describe a novel TFRC humanized mouse model (hTFRC), developed to enable assessment of compounds targeting human TFRC and aiming to deliver drug through the BBB. hTFRC model was developed by knock-in at the mouse endogenous locus to enable a physiological expression of TFRC. Splenocytes from hTFRC mice stimulated with labelled pHrodo Red human transferrin show internalization of human transferrin, suggesting that humanization of the transferrin receptor does not change the endocytosis process. In vivo, treatment of hTFRC mice with anti-BACE1/anti-TFRC antibodies enables the transfer of anti-BACE1 to the brain, while anti-BACE1 antibody is not. As a consequence of the drug delivery, mice treated with anti-BACE1/anti-TFRC show a marked reduction in Amyloid-β 1-40 levels in the brain. Altogether, the data suggest that hTFRC is expressed in the BBB and enables the efficient shuttling of therapeutic antibodies to the brain. While assessment of TFRC-targeting antibodies in a glioma model remains to be performed, the hTFRC model is a promising tool to assess delivery and efficacy of immunotherapies in gliomas. Moreover, the hTFRC model is also being intercrossed with the well-established hSA/FcRn humanized mouse model (Viuff et al., 2016), to enable a more translatable PK and biodistribution of compounds targeting TFRC.

Scientific excellence
From model design to experimental results
Featured in 600+ scientific articles

Collaborative approach
Collaboration with 17 Top Pharmas,
170+ Biotechs and 380+ Academic Institutions

Robust validation data on catalog models
Generated with biopharma partners and in-house

Innovative technologies
and guaranteed freedom to operate

Easy access to models
Models with certified health status from professional breeders in US and Europe