The role of the cGAS-STING pathway for activating myeloid cells to restore antitumor activity

Ren et al. cGAS-STING signaling in the tumor microenvironment induces myeloid cell activation and favors T cell-mediated antitumor immunity, Cancer Biology & Therapy, 2025
The cGAS-STING pathway has emerged as a pivotal innate immune sensor that bridges innate and adaptive immunity by promoting type I interferon (IFN) responses (Sun et al. 2013; Wu et al. 2013; Chen et al. 2016). Its activation within the tumor microenvironment (TME) can overcome immune exclusion, a phenomenon in which cytotoxic T cells fail to penetrate tumor tissue and remain confined to the surrounding stroma, thereby limiting their antitumor activity. This barrier is a major obstacle to effective immunotherapy in cancers such as pancreatic ductal adenocarcinoma (PDAC), which is characterized by dense stroma and profound immunosuppression (Karamitopoulou 2019). While STING agonists have shown promise in preclinical models, their clinical translation remains challenging due to systemic toxicity (Amouzegar et al. 2021). Understanding the cellular and molecular consequences of STING activation in the TME could therefore help designing strategies that maximize antitumor immunity while minimizing adverse effects.
STING agonism reprograms the tumor microenvironment
Building on this rationale and to examine the impact of STING activation in a tumor context, Ren et al. (2025, Cancer Biology & Therapy) used a syngeneic PDAC model (IFNβ⁺/△β-luc B6 mice implanted with KPC tumor cells) combined with single-cell transcriptomics. Treatment with the pyrazole-based STING agonist, EXAC01, delayed tumor growth and triggered strong type I IFN responses, alongside increased infiltration of dendritic cells, monocytes, and CD8⁺ T cells. The study suggests that myeloid cells (DCs, monocytes) are the primary responders to STING activation, driving IFN signaling and antigen presentation, which in turn promote T cell activation. Collectively, these findings highlight the capacity of STING agonists to convert “cold” tumors into inflamed, immune-responsive environments (Corrales et al. 2015; Ager et al. 2021).
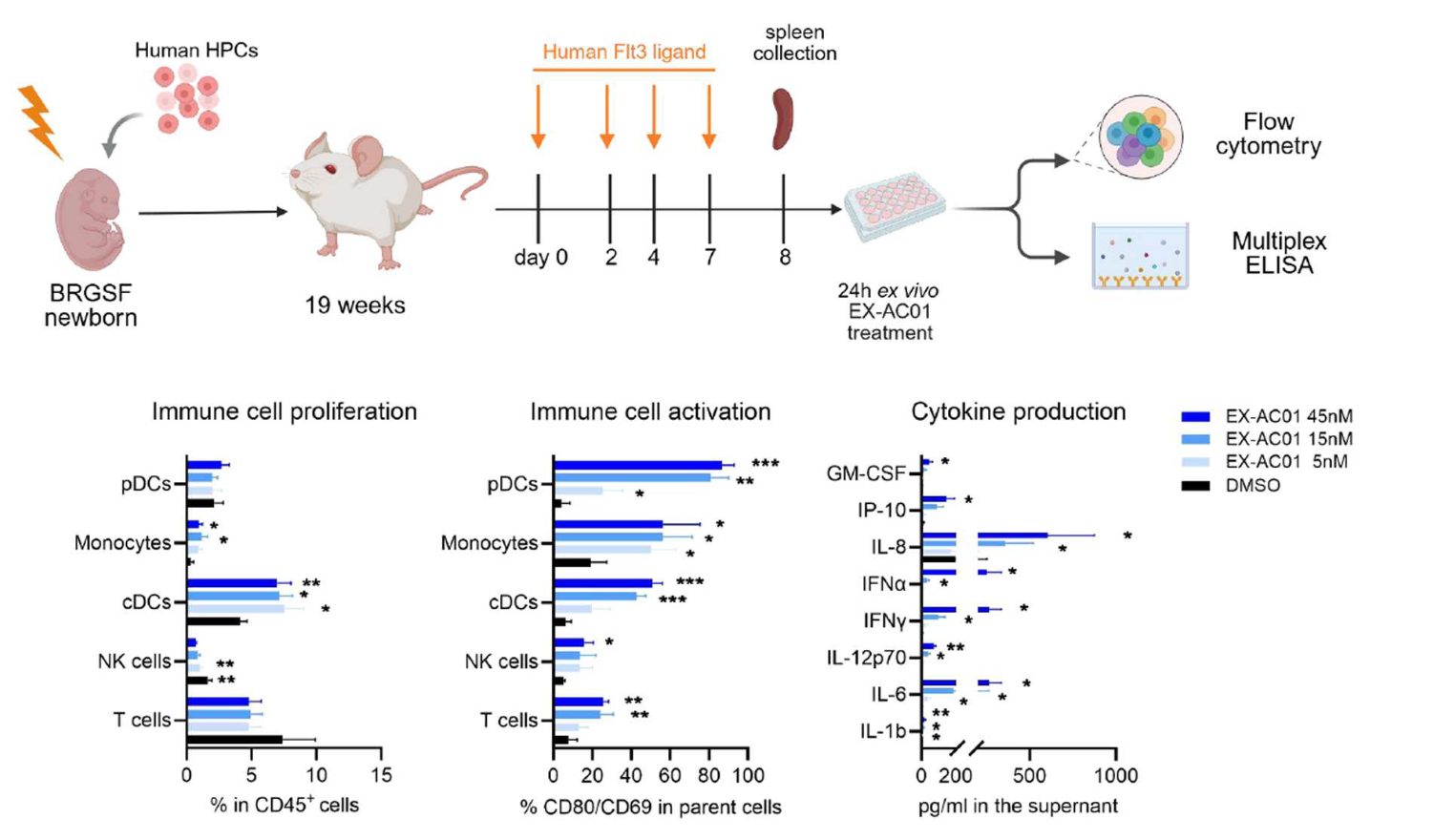
Validating STING-driven mechanisms in a Human Immune System context
To validate the STING-driven mechanisms identified in mouse tumors in a human immune system context and strengthen the translational relevance of their observations, the authors turned to the genO-BRGSF-HIS mouse model, engrafted with hCD34+ hematopoietic stem cells and containing a human immune system. Splenocytes from genO-BRGSF-HIS mice treated with EX-AC01 exhibited increased activation markers (CD69, CD80) and cytokine secretion (IFNβ, IP-10) (Figure 1), confirming that STING agonism can stimulate human myeloid and lymphoid compartments, as previously reported (Martin et al. 2025). Additionally, this paper further confirms that this model can be used for investigating therapies that target the cGAS/STING pathway (Martin et al. 2025). Due to its functional myeloid and lymphoid compartments, this model provides a unique platform to evaluate human-specific immune responses and optimize therapeutic strategies, addressing key challenges in clinical translation.
References
- Ager, Casey R., Akash Boda, Kimal Rajapakshe, et al. 2021. ‘High Potency STING Agonists Engage Unique Myeloid Pathways to Reverse Pancreatic Cancer Immune Privilege’. Journal for ImmunoTherapy of Cancer 9 (8): e003246. https://doi.org/10.1136/jitc-2021-003246.
- Amouzegar, Afsaneh, Manoj Chelvanambi, Jessica Filderman, Walter Storkus, and Jason Luke. 2021. ‘STING Agonists as Cancer Therapeutics’. Cancers 13 (11): 2695. https://doi.org/10.3390/cancers13112695.
- Chen, Qi, Lijun Sun, and Zhijian J. Chen. 2016. ‘Regulation and Function of the cGAS–STING Pathway of Cytosolic DNA Sensing’. Nature Immunology 17 (10): 1142–49. https://doi.org/10.1038/ni.3558.
- Corrales, Leticia, Laura Hix Glickman, Sarah M. McWhirter, et al. 2015. ‘Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity’. Cell Reports 11 (7): 1018–30. https://doi.org/10.1016/j.celrep.2015.04.031.
- Karamitopoulou, Eva. 2019. ‘Tumour Microenvironment of Pancreatic Cancer: Immune Landscape Is Dictated by Molecular and Histopathological Features’. British Journal of Cancer 121 (1): 5–14. https://doi.org/10.1038/s41416-019-0479-5.
- Martin, Gaëlle H., Siham Hedir, Florent Creusat, et al. 2025. ‘Tumor-Dependent Myeloid and Lymphoid Cell Recruitment in genO-BRGSF-HIS Mice: A Novel Tool for Evaluating Immunotherapies’. Frontiers in Immunology 16 (September): 1624724. https://doi.org/10.3389/fimmu.2025.1624724.
- Ren, Meiqi, Zhichao(Eric) Ai, Yan Zhang, et al. 2025. ‘cGAS-STING Signaling in the Tumor Microenvironment Induces Myeloid Cell Activation and Favors T Cell-Mediated Antitumor Immunity’. Cancer Biology & Therapy 26 (1): 2585562. https://doi.org/10.1080/15384047.2025.2585562.
- Sun, L., J. Wu, F. Du, X. Chen, and Z. J. Chen. 2013. ‘Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway’. Science 339 (6121): 786–91. https://doi.org/10.1126/science.1232458.
- Wu, J., L. Sun, X. Chen, et al. 2013. ‘Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA’. Science 339 (6121): 826–30. https://doi.org/10.1126/science.1229963.

Scientific excellence
From model design to experimental results
Featured in 600+ scientific articles

Collaborative approach
Collaboration with 17 Top Pharmas,
170+ Biotechs and 380+ Academic Institutions

Robust validation data on catalog models
Generated with biopharma partners and in-house

Innovative technologies
and guaranteed freedom to operate

Easy access to models
Models with certified health status from professional breeders in US and Europe
