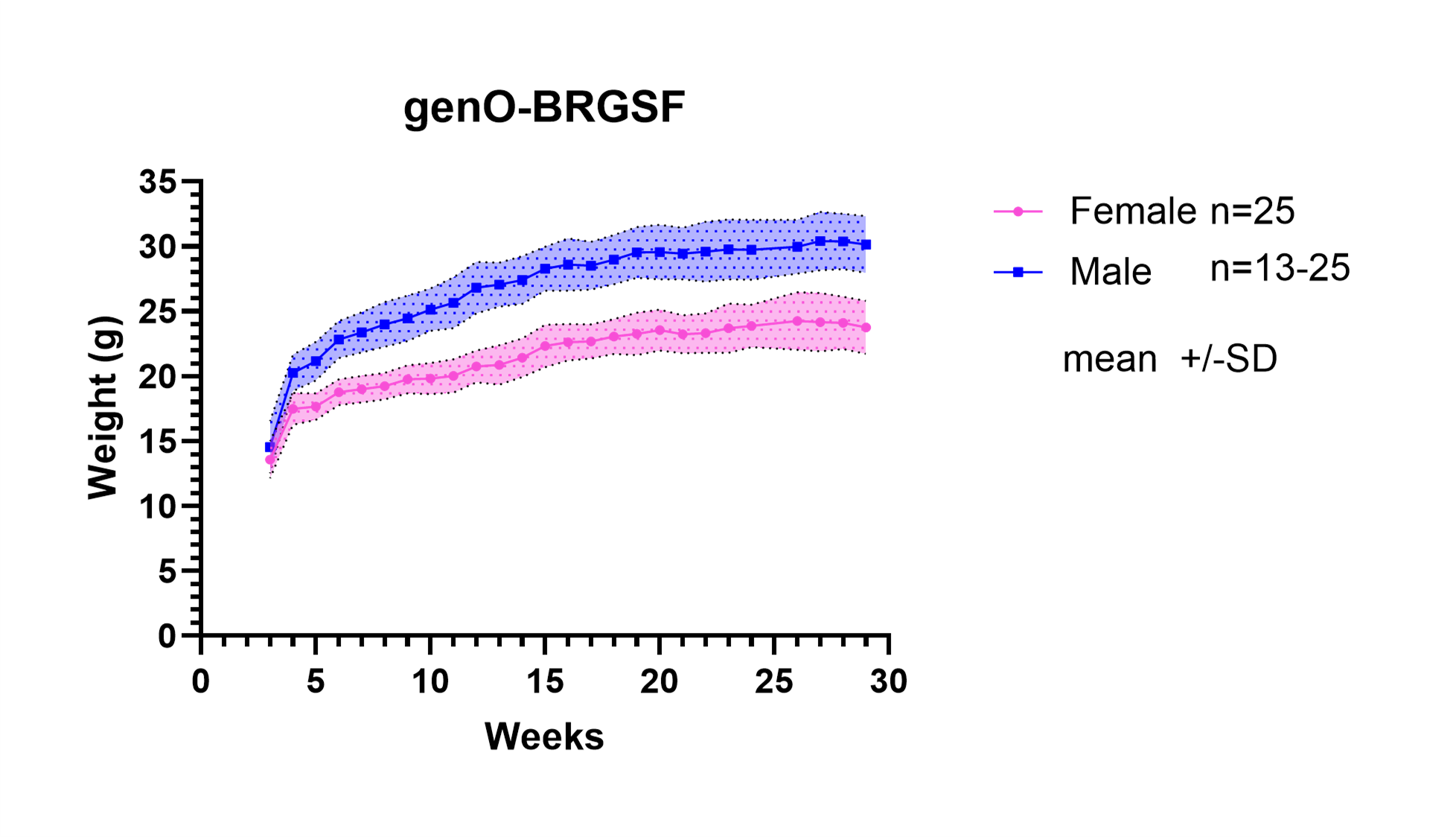
Compared to traditional immunodeficient strains such as NOD-SCID and NSG, the genO‑BRGSF mouse represents the most immunodeficient model generated to date, with defective murine lymphoid and myeloid compartments, and therefore it represents a highly adapted animal model for xenografting of human tumors and/or the immune system.
Applications of the genO‑BRGSF mouse model
The genO‑BRGSF mouse is a unique preclinical model to study:
- Tumor xenografts (including cell-derived xenografts, CDX, and patient-derived xenografts, PDX)
- Evaluation of “standard of care” therapies (surgery, chemotherapy, radiotherapy, etc.)
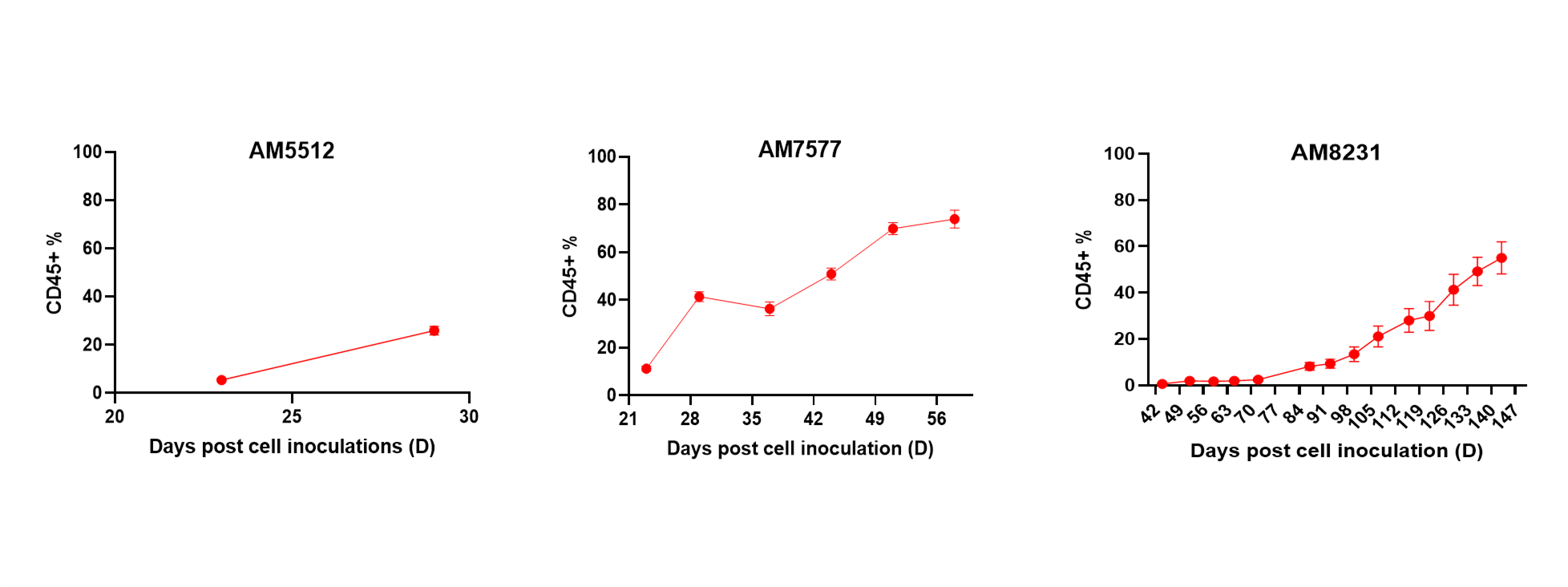
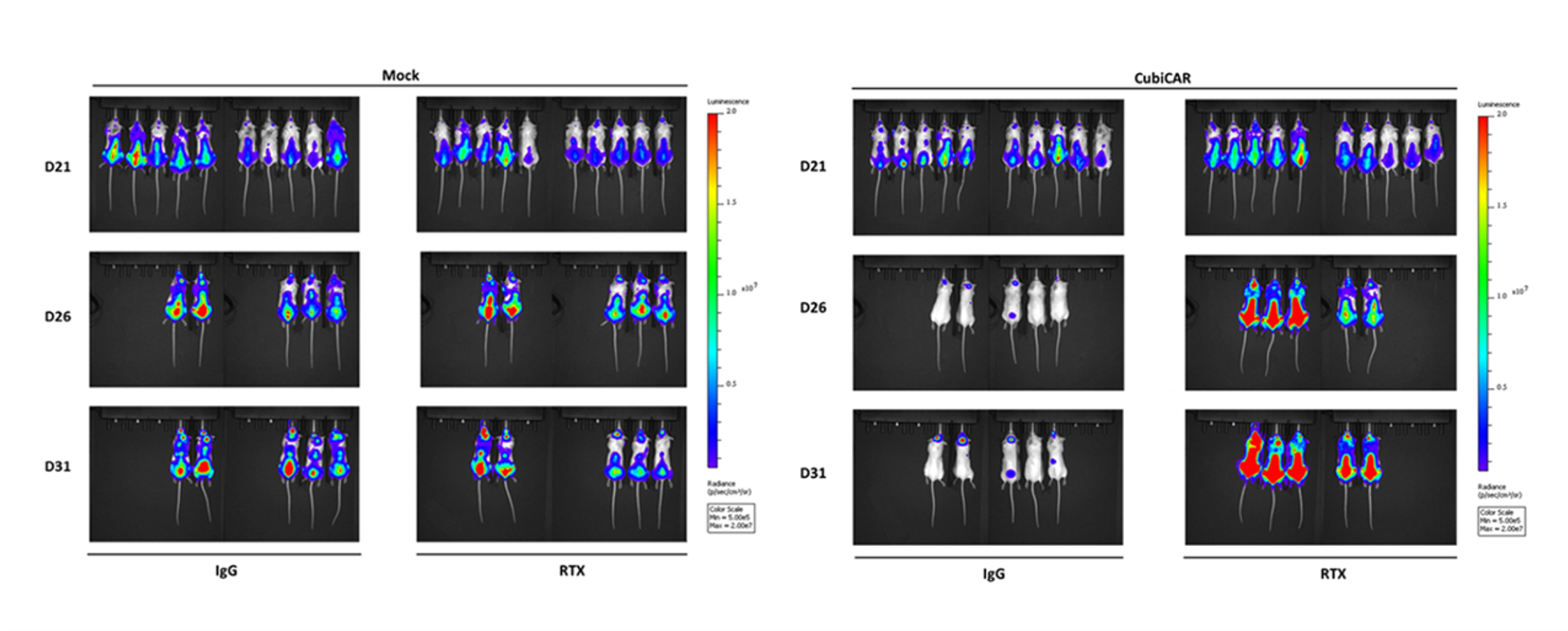
- Cell therapies (including NK, CAR-T cell) efficacy and depletion assessment
- Human CD34+ hematopoietic stem cell engraftment
- Human PBMC cell engraftment
- Complement-dependent cytotoxicity (CDC) studies
Features
- Highly immunodeficient strain lacking murine B and T cells and NK cells, with reduced effector function of murine macrophages due to the mutation in the SIRPα gene, and a significant reduction in the myeloid cell compartment due to a mutation in the FLT3 gene
- Absence of immune cell leakiness or incidence of spontaneous tumors
- Functional complement system
- Extended lifespan and maintenance of a stable phenotype
- Robust model with high tolerability to tumor engraftment
- Resistant to higher levels of radiation and radiation-induced DNA damage due to the presence of the wild-type PRKDC gene

Clients

.png)


























.webp)















.webp)